Research Articles
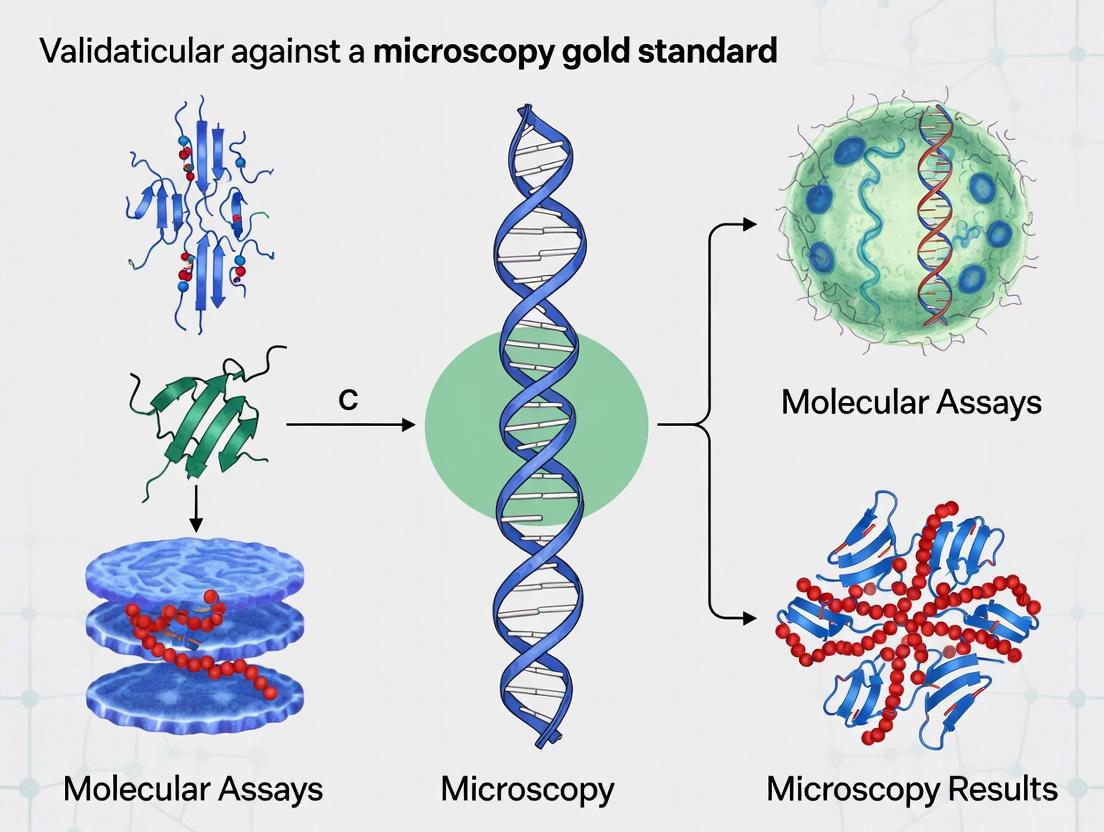
Beyond the Microscope: A Comprehensive Framework for Validating Molecular Assays Against Microscopy Gold Standards
This article provides researchers, scientists, and drug development professionals with a structured guide for the analytical validation of molecular diagnostic tests against traditional microscopy.
Cross-Reactivity in Immunoassays: Advanced Strategies for Detection, Troubleshooting, and Validation in Research and Drug Development
This article provides a comprehensive guide for researchers and drug development professionals confronting the pervasive challenge of cross-reactivity in immunoassays.
Preserving Parasite Integrity: Advanced Strategies to Prevent Specimen Deterioration in Biomedical Research
This article provides a comprehensive guide for researchers, scientists, and drug development professionals on preventing specimen deterioration in parasitology.
Standardizing Sample Collection and Storage: 2025 Best Practices for Research and Drug Development
This guide provides a comprehensive framework for standardizing sample collection and storage, critical for ensuring data integrity and reproducibility in biomedical research and drug development.
Overcoming False Negatives in Dientamoeba fragilis PCR: A Research-Focused Guide to Enhanced Detection and Assay Validation
Molecular diagnostics, particularly PCR, have revolutionized the detection of Dientamoeba fragilis but are susceptible to false-negative results that can impede research and drug development.
Optimizing Primer Templates for Trichomonad Detection: A Strategic Guide for Molecular Assay Development
Accurate detection of Trichomonas vaginalis is critical for addressing the global burden of this prevalent non-viral sexually transmitted infection.
Advancing Cryptosporidium Detection: Strategies for Enhanced Sensitivity in Clinical and Research Settings
Cryptosporidium, a significant cause of diarrheal disease, has been historically underestimated due to the insensitivity of conventional diagnostic methods.
Overcoming PCR Inhibition in Stool Samples: A Comprehensive Guide for Robust Molecular Diagnostics
Accurate molecular analysis of stool samples is critical for clinical diagnostics, gut microbiome research, and drug development.
Breaking the Wall: Advanced Strategies for DNA Extraction from Robust Parasite Oocysts
Efficient DNA extraction from robust parasite oocysts, such as Cryptosporidium and Giardia, is a critical bottleneck in molecular diagnostics and research.
FEA Stool Concentration: Enhanced Detection of Intestinal Parasites in Clinical and Research Settings
This article provides a comprehensive analysis of the Formalin-Ethyl Acetate (FEA) concentration technique for stool specimen examination, a critical diagnostic tool for intestinal parasitic infections (IPIs).